Read up on our continued efforts to develop next-generation batteries to complement renewable power generation…
Published in Nature Chemistry
We are excited to announce that Tom Guarr and Charley Hengesbach from the OESLab have been published in Nature Chemistry! This is part of a collaboration with David Hickey from MSU and Jolt Energy Storage. Better solubility of our electrochemical compounds = better batteries!
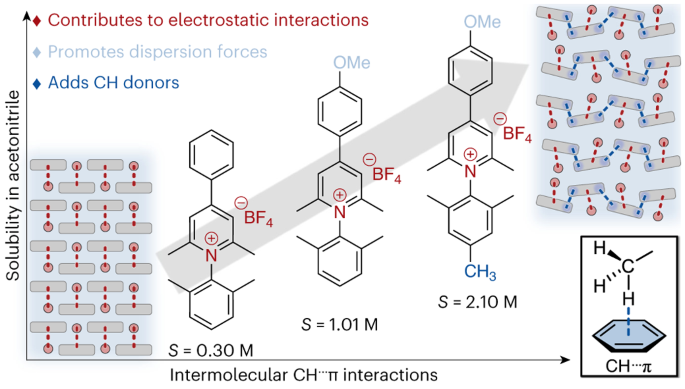
C–H···π interactions disrupt electrostatic interactions between non-aqueous electrolytes to increase solubility
Abstract
Grid-scale energy storage applications, such as redox flow batteries, rely on the solubility of redox-active organic molecules. Although redox-active pyridiniums exhibit exceptional persistence in multiple redox states at low potentials (desirable properties for energy storage applications), their solubility in non-aqueous media remains low, and few practical molecular design strategies exist to improve solubility. Here we convey the extent to which discrete, attractive interactions between C–H groups and π electrons of an aromatic ring (C–H···π interactions) can describe the solubility of N-substituted pyridinium salts in a non-aqueous solvent. We find a direct correlation between the number of C–H···π interactions for each pyridinium salt and its solubility in acetonitrile. The correlation presented in this work highlights a consequence of disrupting strong electrostatic interactions with weak dispersion interactions, showing how minimal structural change can dramatically impact pyridinium solubility.
Congrats to Dr. Guarr, Dr. Hickey, Sharmila Samaroo and Charley Hengesbach!
Summer 2023 intern program success!
It has been a while since we updated this website, but the summer is so busy!
Our lab hosted four new summer interns and a returning intern this summer. I will highlight each one of them in subsequent posting soon. The summer was a whirlwind as always and I will attempt to document some of it with the few pictures I took.
We had a great summer group! Everyone was very supportive and a lot of research was completed
Not only did our group spend time in the lab, but we enjoyed tours of a local brewery, power plant and manufacturer.
We found a little time to get out and enjoy the summer together, but it is never enough.
Please stay tuned for more highlights of each intern in the coming weeks.
Summer 2023 Internship applications now open
We are very excited to open the application process for Summer 2023!
Lots of info here:
- Summer researchers work on individual, unique projects.
- They are supervised by Dr. Tom Guarr, Director of R&D at the Michigan State University Bioeconomy Institute and PI of the OESLab.
- Please note the lab is in Holland, MI, not East Lansing, MI.
- Our summer program lasts ten weeks. This may be extended by mutual agreement.
- Start dates are flexible.
- We are paying $15.00/hr for 40 hours each week.
- We will help you find housing for the summer. Housing may be in a rental with other students.
- At the end of the summer we will participate in a poster session where your individual research can be presented. Please see below for areas of research.
- Look through this blog to see other summer’s research groups and activities.
Please read this post carefully and follow directions to apply.
Go to careers.msu.edu. Search for job # 832628. Follow instructions to apply.
Please include with your application:
- Resume
- Cover letter detailing which area of our research most interests you (see below)
- List of technical classes (You can send your transcript, but all we need is a list)
- At least one reference (preferably academic or research reference)
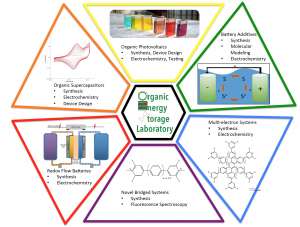
Here is a graphic of our research areas:
Please email Laura Ives with any questions. <—— that is me. I am very responsive.
Our application window will close February 28, 2023.
We are looking forward to hearing from you!
ACS Chicago
Welcome back Dr. Rachel Beltman
We have the honor of welcoming back Rachel Beltman. She started with us as an intern in the summer of 2017. From there, she attended graduate school at Wayne State University and was awarded a PhD in Biochemistry this summer. She is back to help us out with some compounds of interest. Her contributions are much appreciated.
How it started (2017)… How it’s going (2022)….
Rachel’s time back with us was short but sweet. Congratulations on your new position at Gentex. Your future is bright!
Charley Hengesbach
Charley started with the OESLab the summer of 2021 and stayed through the year. She did great research and increased our knowledge of our target compounds immensely. She also did an amazing job working with five new interns this summer.
Charley is continuing to work at MSUBI, but she has moved to the pilot plant area where she will be a Process Engineer. This is great because we still get to see her every day. Thanks Charley!
It was a great summer…
Interested in working for us?
We are hiring researchers! Are you excited about some good organic synthesis and electrochemistry? Are you interested in energy storage, flow batteries and organic chemistry? Please apply!
https://careers.msu.edu/en-us/job/512268/technical-aide
Your application will come straight to us. These jobs are located in Holland, MI. We are looking for Post-bac or Post-doc candidates.
This position will perform original research in energy storage involving organic synthesis, analytical chemistry, electrochemistry and device design. Researchers will work in collaboration with Jolt Energy Storage and NRELaboratory toward battery development. They will also assist with the lab’s summer internship program, working with undergraduate interns and their research projects.

Have questions? Email iveslaur@msu.edu.