Ferrocene- based donor-acceptor systems
Taylor D. Opolka, Dennis Downing and Dr. Thomas Guarr
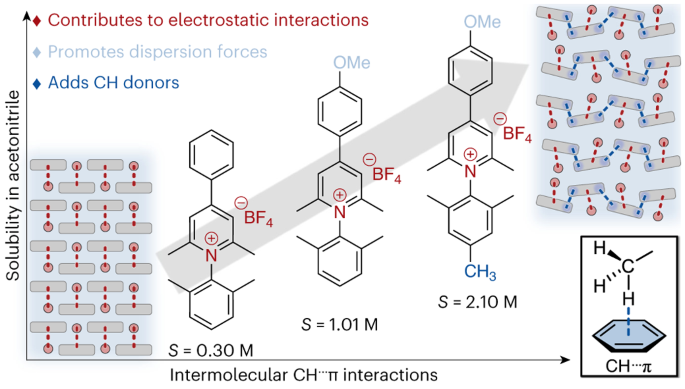
Redox Flow Batteries (RFBs) are preferred over traditional lithium-ion cells for grid storage applications based on increased cycle longevity and safety. However, the cell voltage of RFBs is limited by the low potential window of aqueous solvents and a relatively small library of active materials. Typical RFB design also requires the use of ion-selective membranes to prevent the mixing of anolyte and catholyte, which in turn increases cost. Employing non-aqueous solvents allows much higher cell voltages, but ion-selective membranes are highly resistive when employed in aqueous solvents. Previous work in our lab has focused on the use of bifunctional organic molecules composed of covalently lined anolytes/catholytes that possess three stable oxidation states and can use a simple, inexpensive porous separator rather than an ion-selective membrane. This work focuses on slight modifications of the aryl-linked ferrocene donor-pyridinium acceptor systems in order to improve solubility in inducing molecular asymmetry.
Taylor returned to the lab this summer after finishing up at Davenport and earning her degree and we were really happy to have her back. She is starting a PhD program at Notre Dame this fall that we have no doubt she is going to do great at. Thanks for spending the summer with us again Taylor!