We are excited to announce that Tom Guarr and Charley Hengesbach from the OESLab have been published in Nature Chemistry! This is part of a collaboration with David Hickey from MSU and Jolt Energy Storage. Better solubility of our electrochemical compounds = better batteries!
C–H···π interactions disrupt electrostatic interactions between non-aqueous electrolytes to increase solubility
Abstract
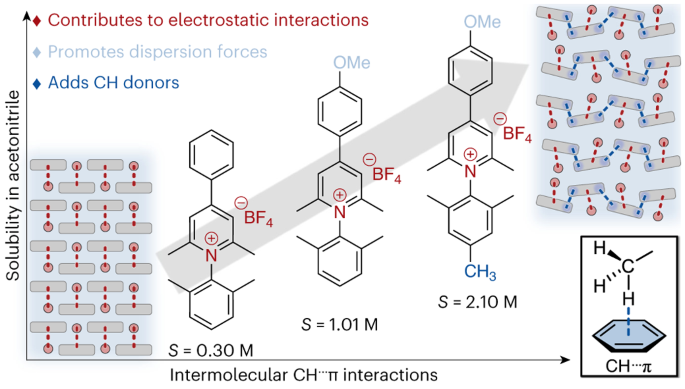
Grid-scale energy storage applications, such as redox flow batteries, rely on the solubility of redox-active organic molecules. Although redox-active pyridiniums exhibit exceptional persistence in multiple redox states at low potentials (desirable properties for energy storage applications), their solubility in non-aqueous media remains low, and few practical molecular design strategies exist to improve solubility. Here we convey the extent to which discrete, attractive interactions between C–H groups and π electrons of an aromatic ring (C–H···π interactions) can describe the solubility of N-substituted pyridinium salts in a non-aqueous solvent. We find a direct correlation between the number of C–H···π interactions for each pyridinium salt and its solubility in acetonitrile. The correlation presented in this work highlights a consequence of disrupting strong electrostatic interactions with weak dispersion interactions, showing how minimal structural change can dramatically impact pyridinium solubility.
Congrats to Dr. Guarr, Dr. Hickey, Sharmila Samaroo and Charley Hengesbach!