Monthly Archives: August 2022
Evelyn Widmaier
Evelyn is a Chemistry major at University of Michigan…
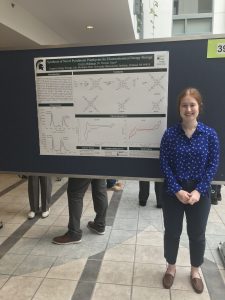
Synthesis of Novel Pyridinium Porphyrins for Electrochemical Energy Storage
Evelyn Widmaier
Dr. Thomas Guarr
Organic Energy Storage Laboratory at Michigan State University Bioeconomy Institute
Porphyrins have applications in nonaqueous redox flow batteries (RBFs) as bipolar redox-active materials and in solar photovoltaic cells as light absorbers and transfer agents. Use of porphyrins in RBFs has been limited by poor reversibility in the second electron redox reaction and low solubility. Pyridinium porphyrins have the potential to improve on these limitations for RBFs. Pyridinium porphyrins may also be viable for photochemical electron cascade in solar photovoltaic cells due to their increased electron-hole pair separation. Zinc-metalated and free base pyridinium porphyrins were obtained by reaction of aminophenyl porphyrin with pyrylium in a multistep synthesis. Redox potentials were characterized by cyclic voltammetry. Here, we present a synthetic pathway for pyridinium porphyrins and ongoing analysis of pyridinium porphyrin usefulness in electrochemical energy storage.
Thanks Evelyn! It was a great summer with you. Enjoy Ann Arbor and UMich.
Taylor Opolka
Taylor is a Biological Laboratory Science major at Davenport University…
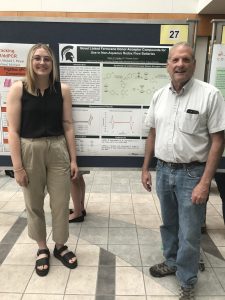
Novel Linked Ferrocene Donor-Acceptor Compounds for Use in Symmetric Non-Aqueous Redox Flow Batteries
Taylor Opolka
Dr. Thomas Guarr
Organic Energy Storage Laboratory at Michigan State University Bioeconomy Institute
Traditional aqueous redox flow batteries are common vessels used in energy storage, but their cell voltage is limited by the potential window of water. Shifting to non-aqueous cells provides a larger potential window and a wider range of active materials that can be utilized. Additionally, employing bifunctional molecules helps mitigate the cost and environmental impact of such cells. Novel linked ferrocene donor-acceptor compounds show great promise for use in these non-aqueous cells based on their physical properties, including good stability, high solubility, and an attractive cell voltage. Two phenyl-linked ferrocene-pyrylium compounds have been synthesized and characterized via mass spectroscopy, nuclear magnetic resonance, and cyclic voltammetry, and have exhibited favorable properties for use in nonaqueous redox flow batteries
Taylor was with us for what may be a record of 16+ weeks of internship. We enjoyed having you Taylor. Keep in touch your last semester at Davenport.
Kendra Hagey
Kendra Hagey is a Chemical Engineering student at University of Michigan…
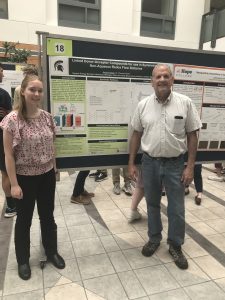
Linked Donor-Acceptor Compounds for use in Symmetric Non-Aqueous Redox Flow Batteries
Kendra Hagey
Dr. Thomas Guarr
Organic Chemistry Storage Laboratory at Michigan State Bioeconomy Institute
As the need for renewable energy grows, inexpensive and reliable grid storage is necessary to meet the energy demand and stabilize the grid against the intermittency of renewable energy sources like wind and solar. Non-aqueous redox flow batteries offer a higher cell voltage than aqueous flow batteries, as the latter are limited by the narrow potential window of water. However, non-aqueous redox flow batteries face other challenges such as reduced conductivity, higher electrolyte cost, and low solubility. Linking the anolyte and the catholyte together in a symmetric system eliminates the need for a costly ion selective membrane and enables the use of a simple porous membrane to facilitate ion transfer. Various donor-acceptor compounds have been synthesized and characterized using mass spectrometry and cyclic voltammetry. Preliminary tests were conducted to determine electrochemical properties, stability, and solubility.
This research was supported by Lakeshore Advantage.
It was a great summer Kendra. Keep in touch while you continue your studies at UMich.
Luke Cooper
Luke is a Chemistry major at Alma College…
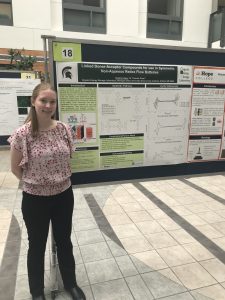
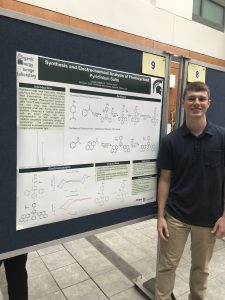
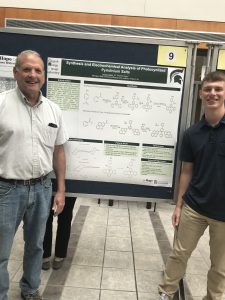
Synthesis and Electrochemical Analysis of Photocyclized Pyridinium Salts
Lucas Cooper
| Pyridinium salts have many uses, including playing a key role in reaching drug targets, food preservatives, and electrolytes for energy storage. Photocyclized pyridinium salts haven’t been explored as thoroughly but may keep their electrochemical properties while also having other useful qualities. Analyzing a cyclized pyridinium with an amino group may show polymerization at high voltages and catalytic properties previously unseen in pyridiniums. The compounds were synthesized from a pyrylium intermediate, followed by a condensation, and exposure to oxygen and ultraviolet light. The compounds were examined using mass spectrometry, and cyclic voltammetry. |
Thanks for the summer Luke! Have a great last year at Alma.
Brett Cesar
Our first highlighted intern is Brett Cesar. Brett is a Chemical Engineering student at MSU…
Stability Analysis of Non-Aqueous Redox Flow Battery Active Materials
Brett Cesar
Dr. Thomas Guarr
Organic Chemistry Storage Laboratory at Michigan State Bioeconomy Institute
Redox flow batteries offer the benefit of being safer and more scalable than traditional lithium-ion batteries. However, most redox flow batteries make use of aqueous solvents that limit the cell voltage and employ relatively rare metals that increase the cost and environmental impact of the cell. Non-aqueous redox flow batteries have been explored to reduce these costs while increasing cell voltage and energy density. One method of achieving these goals includes the use of linked active materials in symmetric, non-aqueous redox flow batteries. The stability of several linked compounds prepared in our lab was analyzed through spectroelectrochemistry and bulk electrolysis.
This research was supported by the Community Foundation of Holland/Zeeland, Lakeshore Advantage, and Michigan State University.
Thanks for spending the summer with us. Good luck with the next triathlon. Wish we could keep up!