A graduate of University of Detroit Mercy, with a degree in Biochemistry, Rachel synthesized many compounds for us this summer.
Extended Bispyridines: an Approach to Molecular Wires; Rachel Beltman and Dr. Thomas Guarr
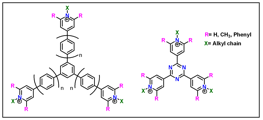
Recently, redox flow batteries (RFBs) have been intensively researched for grid-level energy storage applications. In addition to offering an inexpensive option for long term storage, this technology also provides a means to achieve the load leveling required to effectively utilize renewable sources of energy. Bispyridinium compounds are of interest in RFBs because they offer reasonable voltages, good stability, and relatively high energy densities. We have developed a series of extended bispyridinium systems that incorporate p-phenylene (or longer) bridges can also be used as anolyte materials.
The extension of the π system in such compounds allows for an increase in cell voltage, along with a corresponding improvement in energy density. Stability is also improved because the ability to accept two electrons and achieve a stable, closed shell reduced state helps to avoid the buildup of less stable radical intermediates. In this study, the effects of substituent choice and bridge length on cell potential, molecular weight, durability, and ease of synthesis are explored.

Rachel has proven herself to be quite talented at organic synthesis, and she is continuing her studies at Wayne State this fall to pursue a doctorate in Organic Chemistry. We appreciate all of your contributions this summer, Rachel. Enjoy your time at Wayne State.