The final poster highlighted is Kassia’s…
Stability testing of radical cations for energy storage applications.
Kassia Symstad, Dr. Thomas F. Guarr
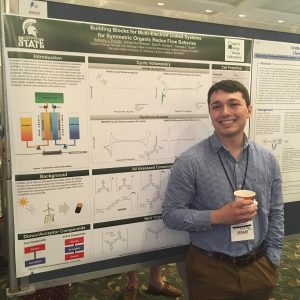
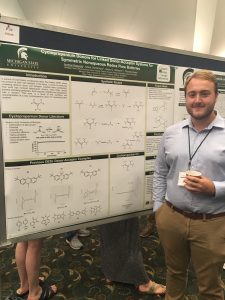
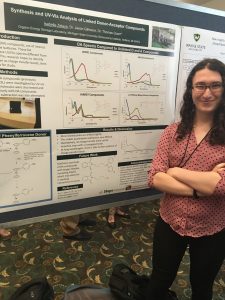
Electron donor molecules must be stable in both the neutral and radical cation form to be viable as shuttles for overcharge protection in lithium ion batteries or as part of a larger donor-acceptor system for use in redox flow batteries. In this work we report stability testing on a variety of carbazole and phenothiazine-5,5-dioxide derivatives. Conversion of the neutral molecule to its radical cation state was accomplished either by chemical oxidation or electrolysis in a spectroelectrochemical cell. The radical cations of these donor molecules have a differect UV-vis spectra than the neutral molecules, which provides an effective means to monitor their generation and persistence. The voltage that resulted in the maximum rate of conversion to the radical cation form was found this way. that voltage was them applied for a longer period of time, either in a spectroelectrochemical cell or a conventional H-cell for bulk electrolysis to effect complete conversion. This allows the radical cation to be studied on time scales ranging from seconds to months. These stability studies are useful for the evaluation of preliminary data from a linked donor-acceptor system in a prototype RFB cell.